User:Milton Beychok/Sandbox
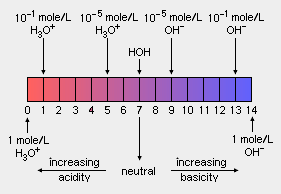
The pH scale measures the acidity or alkalinity of an aqueous solution. Values for pH range from 0 (strongly acidic) to 14 (strongly alkaline or basic). The pH of a neutral solution (neither acid or basic), such as pure water at room temperature and atmospheric pressure is 7, whereas the pH of an acidic solution is less than 7 and the pH of a basic solution is greater than 7. The pH scale is logarithmic which means that a difference of one pH unit is equivalent to a ten-fold difference in hydrogen ion concentration. The notation pH is sometimes referred to as the power of hydrogen or the potential of hydrogen.
The traditional way to determine whether a solution is acidic or basic is by wetting litmus paper with the solution. If the wet litmus paper turns red, the solution has a pH less than 7 and is acidic. If it turns blue, the solution has a pH greater than 7 and is acidic. Measuring the actual pH value of a solution is more accurately done with a pH meter.
The pH scale was originally defined by Danish biochemist Søren Peter Lauritz Sørensen in 1909, who wrote it as PH. It was subsequently changed to the modern notation of pH in 1920 by William Mansfield Clark, an American biochemist, for typographical convenience in his book The Determination of Hydrogen Ions.[1][2]
Formal definition
The concentration of hydrogen ions (in moles per litre of solution) is written simply as [H+] or as hydronium [H3O+] and both describe the same entity. The pH value is defined by taking the logarithm of this concentration:
Since the product of the concentration of hydronium ions and the concentration of hydroxide ions is constant, namely
taking logarithms gives
where pOH is defined in a manner similar to pH, as shown below.
The pH scale measures the acidity or alkalinity of an aqueous solution. Values for pH range from 0 (strongly acidic) to 14 (strongly alkaline or basic). The pH of a neutral solution (neither acid or basic), such as pure water at room temperature and atmospheric pressure is 7, whereas the pH of an acidic solution is less than 7 and the pH of a basic solution is greater than 7. The pH scale is logarithmic which means that a difference of one pH unit is equivalent to a ten-fold difference in hydrogen ion concentration. The notation pH is sometimes referred to as the power of hydrogen or the potential of hydrogen.
The traditional way to determine whether a solution is acidic or basic is by wetting litmus paper with the solution. If the wet litmus paper turns red, the solution has a pH less than 7 and is acidic. If it turns blue, the solution has a pH greater than 7 and is acidic. Measuring the actual pH value of a solution is more accurately done with a pH meter.
The pH scale was originally defined by Danish biochemist Søren Peter Lauritz Sørensen in 1909, who wrote it as PH. It was subsequently changed to the modern notation of pH in 1920 by William Mansfield Clark, an American biochemist, for typographical convenience in his book The Determination of Hydrogen Ions.[3][4]
Formal definition
The concentration of hydrogen ions (in moles per litre of solution) is written simply as [H+] or as hydronium [H3O+] and both describe the same entity. The pH value is defined by taking the logarithm of this concentration:
Since the product of the concentration of hydronium ions and the concentration of hydroxide ions is constant, namely
taking logarithms gives
where pOH is defined in a manner similar to pH, as shown below.
pH of some common solutions
|
|
References
- ↑ Willam Mansfield Clark (1920). The Determination of Hydrogen Ions, 1st Edition. William & Wilkins Company, page 35. Available in Google Books here
- ↑ pH: Potenz, The Determination of Hydrogen Ions, History of Analytical Chemistry, Electrochemistry, Past and Present From the JRank Science & Philosophy website
- ↑ Willam Mansfield Clark (1920). The Determination of Hydrogen Ions, 1st Edition. William & Wilkins Company, page 35. Available in Google Books here
- ↑ pH: Potenz, The Determination of Hydrogen Ions, History of Analytical Chemistry, Electrochemistry, Past and Present From the JRank Science & Philosophy website
- "General Chemistry, 2nd Ed.", pp 103-117, D. D. Ebbing & M. S. Wrighton, Houghton Mifflin, Boston, 1987.
- "General Chemistry with Qualitative Analysis, 2nd Ed.", pp. 263-278, Saunders College Publishing, Philadelphia, 1984.
References
- "General Chemistry, 2nd Ed.", pp 103-117, D. D. Ebbing & M. S. Wrighton, Houghton Mifflin, Boston, 1987.
- "General Chemistry with Qualitative Analysis, 2nd Ed.", pp. 263-278, Saunders College Publishing, Philadelphia, 1984.
![{\displaystyle \mathop {\rm {pH}} =-\log _{10}\left[{\rm {H_{3}O^{+}}}\right]=\log _{10}{\frac {1}{\left[{\rm {H_{3}O^{+}}}\right]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fa5bac61469877eb81acca1a88b271a5458614ab)
![{\displaystyle \left[{\rm {H_{3}O^{+}}}\right]\left[{\rm {OH^{-}}}\right]=1.0\cdot 10^{-14}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/26737c368f1e581d3af342fc86472bc21535b63e)

![{\displaystyle \mathop {\rm {pOH}} =-\log _{10}\left[{\rm {OH^{-}}}\right]=\log _{10}{\frac {1}{\left[{\rm {OH^{-}}}\right]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8a32f6bd6cbbbafe18f7aeeca7a44743236c46d0)