Neutron activation analysis
Neutron activation analysis (NAA) is an extremely sensitive technique of radiochemical analysis used to determine the existence and quantities of major, minor and trace elements in a material sample. As opposed to other methods in analytical chemistry, such as mass spectrometry or chromatography, it focuses entirely on the nuclei of atoms, not their molecular structure. It reports the concentrations of all elements in a sample, down to extremely low levels.
Principles
When samples are irradiated with neutrons, certain of their elements become radioactive, and emit gamma rays of various energies. "This emitted radiation is a 'fingerprint' of the element, and the amount of radiation given off at a certain energy is indicative of the amount of the element present in the sample. A comparison between specific activities induced in the standards and unknowns provides the basis for computation of elemental abundances. From this analysis, a report is issued giving elemental concentrations in the unknown sample."[1]
The above description implies a measurement at a single point in time. Taking multiple measurements, of "prompt" and "delayed" gamma emission, adds another dimension. Two given elements might emit gammas of the same energy, but since the radioactive isotopes have different half-lives, one will stop emitting before the other.
Applicability
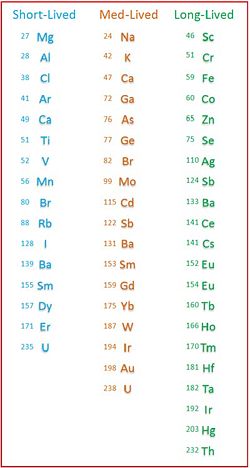
Not all elements absorb neutrons in a manner that makes them detectable by NAA, but over 50 do. Further, they can be further differentiated by the time they stay activated.
Some samples are inappropriate for NAA generally, or for specific facilities. Oak Ridge, for example, warns "Among these are liquids (generally), materials highly corrosive to stainless steel or aluminum, materials that react violently with water, explosives and highly flammable substances, and materials that pose exceptional danger to humans."
The Oak Ridge irradiation container inner dimensions are 10mm × 20mm; other facilities may use other sizes.
Examples of applications from the University of Wisconsin include:
- Detecting impurities in industrial and food products
- Tracing the transport and utilization of elements in animal metabolism
- Looking for arsenic in human hair to determine if someone was poisoned
- Checking soil samples of reclaimed dump sites to see if there were any hazardous materials left over from those sites
- Looking for mercury or arsenic in wild animals that inhabit the Mississippi river area
- Determining trace elements in Native American and Eastern European archeological artifacts to help reconstruct ancient trade routes
- Irradiating dinosaur bones to look for iridium to show that a meteor caused their extinction
General comparison with other methods
Advantages
- Inherently nondestructive. "The sample is not permanently damaged by NAA, and in the case case of forensic analysis and analysis of rare samples, such as meteorites or archeological finds, the sample can be saved and even subjected to further analysis at a later time...the sample may become slightly radioactive in NAA, the radiation in the sample decreases with time until it reaches a state similar to which it was before the NAA was performed on it."
- "detects the total elemental content, regardless of oxidation state, chemical form or physical location." [2]
- Simultaneously detects all elements in the sample
- Time-efficient for analyzing many samples
Disadvantages
There must be a source of neutrons. For some applications, this must be a full-sized nuclear reactor, with all the associated safety and cost consideration. Portable sources, however, work for some applications.
Not all elements are detectable.
Major facilities
Having a nuclear reactor is a prerequisite for the most extensive analytical service, as well as research into NAA. These include:
- High Flux Isotope Reactor at Oak Ridge National Laboratory.[3]
- Nuclear Reactor Laboratory, University of Wisconsin
Applications
Explosives
By examining both ratios of major elements (e.g., carbon, hydrogen, oxygen, nitrogen) as well as the presence of trace elements, NAA, even though explosives are molecules, has been able both to trace the source of explosions after detonation, as well as nondestructively tracing the manufacturer of explosives in situ. [4] NAA has been useful, in like manner, in tracing chemical warfare agents and nuclear materials.
Reference standards are being developed by the National Institute of Standards and Technology. [5], with available samples for RDX, TNT and HMX.
They form part of land mine detection systems, working on the explosive or a plastic casing not sensed by a metal detector.
Forensics
NAA has significant potential in its use in legal forensics, but it must be presented in proper context. From the legal standpoint,
the interpretation of the analysis presents difficult legal problems that depend almost entirely on the circumstances of the individual case. The analytical chemist is qualified to present the results of the analysis, but his subjective interpretation, as a chemist, is worthless; an expert in statistics is required to present a legal interpretation of the analysis. The expert must have a solid, objective basis for any statement beyond the composition of the measured samples, and the burden of laying an objective foundation for the testimony should rest on the party seeking to introduce the results of the tests. The expert must use well-defined terms which fit the legal issue presented by the facts of the case, and must present his testimony in such a way that the trier of fact clearly understands the limitations of the analysis. [6]
Geology
While NAA is useful for trace element analysis in geochemistry, other methods, such as inductively coupled plasma-atomic emission spectrometry (ICP-AES) and atomic emission spectrometry, may be preferred or complementary when measurement of elements with low atomic numbers are needed. [7]
References
- ↑ Neutron Activation Analysis Description, Nuclear Reactor Laboratory, University of Wisconsin
- ↑ Advantages of NAA over other chemical analyses, Nuclear Reactor Laboratory, University of Wisconsin
- ↑ What is Neutron Activation Analysis?, Neutron Sciences, Oak Ridge National Laboratory
- ↑ Nunes WV, da Silva AX, Crispim VR, Schirru R (2002 Jun), "Explosives detection using prompt-gamma neutron activation and neural networks", Appl Radiat Isot. 56(6): 937-43.
- ↑ Development of NIST Standard Reference Materials for Trace Explosives Detection, National Institute of Standards and Technology
- ↑ Dennis S. Karjala, "Comment, the Evidentiary Uses of Neutron Activation Analysis", California Law Review 59 p. 997, 1971
- ↑ Ian Jarvis and Kym E. Jarvis (July 1992), "Inductively coupled plasma-atomic emission spectrometry in exploration geochemistry", Journal of Geochemical Exploration 44, Issues 1-3: 139-200, DOI:10.1016/0375-6742(92)90050-I