Coal
Coal is a carbon-containing rock formed by the debris from the decay of ferns, vines, trees and other plants which flourished in swamps millions of years ago. Over time, the debris became buried and the actions of bacteria, heat and pressure transformed the debris first into peat (a precursor of coal) and then into the various types of coal as we know them today.[1][2][3] In more technical terminology, that process of transformation is referred to as metamorphosis, coalification or lithification.
Coal mining occurs for coal deposits that exist deep underground, as well as one that are at or near the surface of the ground. Because of the various degrees of transformation that occurred during the forming of coal deposits in different locations, the composition of coal varies from one deposit to another. No two coals are the same in every respect. In general, coal consists of carbon, hydrogen, oxygen, nitrogen, sulfur and mineral matter (including compounds of silicon, aluminium, iron, calcium, magnesium and others).
Coal classification
There are many compositional differences between the coals mined from the different coal deposits worldwide. The different types of coal are most usually classified by rank which depends upon the degree of transformation from the original source (i.e., decayed plants) and is therefore a measure of a coal's age.[1][4] As the process of progressive transformation took place, the heating value and the fixed carbon content of the coal increased and the amount of volatile matter in the coal decreased. The method of ranking coals used in the United States and Canada was developed by the American Society for Testing and Materials (ASTM) and is based on a number of parameters obtained by various prescribed tests:
- Heating value: The energy released as heat when coal (or any other substance) undergoes complete combustion with oxygen.
- Volatile matter: The portion of a coal sample which, when heated in the absence of air at prescribed conditions, is released as gases. It includes carbon dioxide, volatile organic and inorganic gases containing sulfur and nitrogen.
- Moisture: The water inherently contained within the coal and existing in the coal in its natural state of deposition. It as measured as the amount of water released when a coal sample is heated at prescribed conditions. It does not include any free water on the surface of the coal. Such free water is removed by air-drying the coal sample being tested.
- Ash: The inorganic residue remaining after a coal sample is completely burned and is largely composed of compounds of silica, aluminium, iron, calcium, magnesium and others. The ash may vary considerably from the mineral matter present in the coal (such as clay, quartz, pyrites and gypsum) before being burned.
- Fixed carbon: The remaining organic matter after the volatile matter and moisture have been released. It is typically calculated by subtracting from 100 the percentages of volatile matter, moisture and ash. It is composed primarily of carbon with lesser amounts of hydrogen, nitrogen and sulfur.
The ASTM ranking system is presented in the table below:
Class or Rank |
Group |
Fixed Carbon (b) (wt % dry mmf) |
Volatile Matter (b) (wt % dry mmf) |
Gross Heating Value (c) (MJ/kg moist mmf) | |||
|---|---|---|---|---|---|---|---|
| Equal or greater than |
Less than |
Greater than |
Equal or less than |
Equal or greater than |
Less than | ||
| Anthracitic |
Metaanthracite (d) Anthracite (d) Semianthracite (d) |
98 92 86 |
98 92 |
2 8 |
2 8 14 |
||
| Bituminous |
Low-volatile bituminous (d) Medium-volatile bituminous (d) High-volatile A bituminous High-volatile B bituminous High-volatile C bituminous (e) High-volatile C bituminous (f) |
78 69 |
86 78 69 |
14 22 31 |
22 31 |
32.55 30.23 26.74 24.41 |
|
| Subbituminous |
Subbituminous A Subbituminous B Subbituminous C |
24.41 22.09 19.30 |
26.74 24.41 22.09 | ||||
| Lignite |
Lignite A Lignite B |
14.65 |
19.30 14.65 | ||||
| (a) This classification does not include a few coals (referred to as unbanded coals) having unusual physical and chemical properties falling within the fixed carbon and heating value ranges of the high-volatile bituminous and subbituminous ranks. (b) Percentage by weight on a dry and mineral matter free basis (mmf). | |||||||
The anthracitic coals, with the highest contents of fixed carbon and lowest contents of volatile material, have the highest rank. The lignite coals, with the lowest contents of fixed carbon and highest contents of volatile matter, have the lowest rank. The bituminous and subbituminous coals (in that order) are ranked between the anthracitic and lignite coal. The diagram below provides the estimated percentage of the world's coal reserves for each coal rank. It also provides the typical uses of each coal rank.
As a broad generality, the anthracitic coals have the highest heating value and the lignite coals have the lowest heating values.
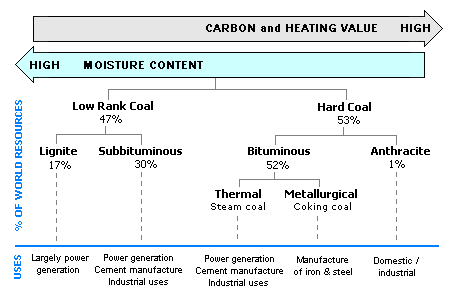
Diagram of the typical uses and the estimated percentage of the world's coal reserves for each coal rank.[6]
There are other coal classification systems developed by the International Organization for Standardization (ISO), the United Kingdom and perhaps others.[4][7]
Coal analysis
The composition of a coal is usually reported in terms of its proximate analysis and its ultimate analysis:
- The proximate analysis consists of four items: fixed carbon, volatile matter, moisture and ash, all on a weight percent basis.
- The ultimate analysis provides an element-by-element composition of the coal's organic fraction, namely: carbon, hydrogen, oxygen and sulfur, all on a weight percent basis.
Both the proximate and the ultimate analysis may be reported on an as received (ar) basis, a dry (d) or moist basis, an ash-free (af) basis, a mineral matter-free (mmf) basis and various combinations of those bases. For example, an analysis may report the basis to be: as received (ar), dry and ash-free (daf), moist and ash-free (maf), dry and mineral matter-free (dmmf) or moist mineral-matter free (moist mmf).
Ash and mineral matter are two distinctly different entities. Mineral matter consists of the various minerals contained in the coal. Ash is the inorganic solids remaining after the coal is completely combusted. The ash is usually less than the mineral matter because of the weight changes that take place during coal combustion such as the loss of gaseous carbon dioxide from mineral carbonates, loss of water from silica minerals and loss of sulfur (as gaseous sulfur dioxide) from iron pyrites.
Some examples of proximate and ultimate analyses are given in the table below:
Coal Rank |
Proximate Analysis (wt % ar) |
Ultimate Analysis (wt % maf) |
Net Heating Value (maf) (MJ/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixed carbon |
Volatile matter |
Moisture |
Ash |
C |
H |
O |
N |
S | |||
| Anthracite | 81.8 | 7.7 | 4.5 | 6.0 | 91.8 | 3.6 | 2.5 | 1.4 | 0.7 | 36.2 | |
| Bituminous | 54.9 | 35.6 | 5.3 | 4.2 | 82.8 | 5.1 | 10.1 | 1.4 | 0.6 | 36.1 | |
| Subbituminous | 43.6 | 34.7 | 10.5 | 11.2 | 76.4 | 5.6 | 14.9 | 1.7 | 1.4 | 31.8 | |
| Lignite | 27.8 | 24.9 | 36.9 | 10.4 | 71.0 | 4.3 | 23.2 | 1.1 | 0.4 | 26.7 | |
| Notes: • wt % = percent by weight ar = as received maf = moisture and ash free | |||||||||||
Coal reserves and production statistics
Economically recoverable coal deposits exist in more than 70 nations and in every major region of the world (Africa, Asia, Australia, Europe, North America and South America). It has been estimated that the worldwide proven reserves of coal amounted to about 848 gigatonnes (Gt) as of 2007.[9] Proven coal reserves are those coal deposits that have been confirmed by exploration, drilling and other means, and which are economically and technically extractable.
It has also been estimated that the worldwide production (i.e., mining) of coal amounted to about 5.54 gigatonnes (Mt) as of 2007.[10] If that rate of production remains constant, the proven reserves will last about 150 years.[9]
The tables below list the distribution of coal reserves and coal production nation-by-nation as of 2007:
|
|
Coal as a fuel
Due to its relatively high carbon content and solid, easily-handled form, coal is used for fuel, and has been for hundreds of years (see history of coal mining). As a fuel, coal is the largest source of energy for the generation of electricity worldwide. In 2005, coal fuelled 40% of the world's electricity generating power plants.[11][12]
A major component of the combustion flue gases produced by burning coal as a fuel is carbon dioxide (CO2), which is not a pollutant in the traditional sense since it is essential to support photosynthesis for all plant life on Earth. However, carbon dioxide is a greenhouse gas considered to be a contributor to global warming. It is the most abundant anthropogenic (human caused) greenhouse gas in the Earth's atmosphere. As shown above, coal may contain from about 70 to more than 90 weight percent carbon, which burns almost completely to carbon dioxide. Hence, coal is the fossil fuel with the largest "carbon footprint".
Currently in the United States
Coal-fired power plants provided about 50 percent of the electric power generated in the United States during 2007.[13] About 92% of the coal mined in the United States is burned to produce electricity.[13][14]
The consumption of coal in the United States by sector (as a percentage of the total coal mined in 2007) was 92.7 % for electric power generation, 2.0 % for production of coke, 5.0 % for use in other industries and 0.3 % for residential and commercial heating.[15]
Currently in China
Coal produces over 80% of China's energy; 2.3 billion metric tons of coal were mined in 2007. Despite the health risks posed by severe air pollution in cities (see Beijing) and international pressure to reduce greenhouse emissions, China’s coal consumption is projected to increase in line with its rapid economic growth. Most of the coal is mined in the western provinces of Shaanxi and Shanxi and the northwestern region of Inner Mongolia. However most coal customers are located in the industrialized southeastern and central coastal provinces, so coal must be hauled long distances on China’s vast but overextended rail network. More than 40% of rail capacity is devoted to moving coal, and the country has been investing heavily in new lines and cargo-handling facilities in an attempt to keep up with demand. Despite these efforts, China has suffered persistent power shortages in industrial centers for more than five years as electricity output failed to meet demand from a booming economy. Demand for electricity increased 14% in 2007.
Other uses of coal
Coal can be converted to coke by the process of destructive distillation which removes the volatile matter and alters the physical properties to provide a more uniform and more combustible product with a higher carbon content.[16] The process is often referred to as the coking or carbonization of coal. One of the major uses of coke is in the making of steel where coke is utilized in blast furnaces to reduce iron ore (iron oxide) to molten pig iron, a form of iron with high carbon content. Removal of most of the carbon from the pig iron yields steel that is used for construction of bridges, high-rise buildings, manufacturing of automobiles and major household appliances (refrigerators, cooking stoves and washing machines), and a host of other products. Coke is also used in the production of phosphorus and of calcium carbide.
Coal can be converted, by a process known as coal gasification, into a gas with the same heat of combustion as many natural gases and referred to as synthetic natural gas (SNG).[17][18] Coal gasification can also be used to produce a mixture of carbon monoxide and hydrogen gases referred to as synthesis gas (or syngas) which has a heat of combustion that is much less than that of natural gas. Syngas can be burned as a fuel or it can be converted into automotive fuels like gasoline and diesel oil through the Fischer-Tropsch process.[19]
Coal mining
See Coal mining for details.
References
- ↑ 1.0 1.1 1.2 1.3 Green, Don W. and Perry, Robert H. (Editors) (1997). Perry's Chemical Engineers' Handbook, 6th Edition. McGraw-Hill. ISBN 0-07-049479-7.
- ↑ 2.0 2.1 Eugene A. Avallone, Theodore Baumeister and Ali Sadegh (Editors) (2006). Marks' Standard Handbook for Mechanical Engineers, 11th Edition. McGraw-Hill Professional. ISBN 0-07-142867-4.
- ↑ 3.0 3.1 Frank Kreith (Editor) (1998). The CRC Handbook of Mechanical Engineering, 1st Edition. CRC Press. ISBN 0-8493-9418-X.
- ↑ 4.0 4.1 J.G. Speight (1994). The Chemistry and Technology of Coal, 2nd Edition, Revised and Expanded. CRC Press. ISBN 0-8247-9200-9.
- ↑ Klaus K.E. Neuendorf, James P. Mehl and Julia A. Jackson (2005). Glossary of Geology, 5th Edition. American Geological Institute. ISBN 0-922152-76-4.
- ↑ Coal Information World Coal Institute
- ↑ Sunggyu Lee (1996). Alternative Fuels, First Edition. CRC Press. ISBN 1-56032-361-2.
- ↑ Chris Higman and Maarten van der Burgt (2008). Coal Gasification, 2nd Edition. Gulf Professional Publishers. ISBN 0-7506-8528-X.
- ↑ 9.0 9.1 9.2 2007 Survey of Energy Resources World Energy Council 2007
- ↑ 10.0 10.1 Coal Facts 2008 Edition (with 2007 data)
- ↑ International Energy Association, 2006, Key Energy Statistics (International Energy Agency)
- ↑ International Energy Outlook 2008: Chapter 5 (Energy Information Administration, U.S. DOE)
- ↑ 13.0 13.1 Summary Statistics for the United States] Electric Power Annual (2007) published by the Energy Information Administration of the U.S. Department of Energy
- ↑ Coal Production and Number of Mines by State and Mine Type Annual Coal Report (2007) published by the Energy Information Administration of the U.S. Department of Energy
- ↑ Annual Coal Report (2007), Executive Summary Published by the Energy Information Administration of the U.S. Department of Energy
- ↑ Many petroleum refineries produce a similar product, called petroleum coke, by a process known as delayed coking.
- ↑ Synthetic Natural Gas (SNG): Technology, Environmental Implications, and Economics Munish Chandel and Eric Williams, Climate Change Policy Partnership, Duke University, January 2009
- ↑ Beychok, M.R., Process and environmental technology for producing SNG and liquid fuels, U.S. EPA report EPA-660/2-75-011, May 1975
- ↑ Coal Gasification & Fischer-Tropsch Brian H. Bowen and Marty W. Irwin, The Indiana Center for Coal Technology Research, Purdue University, July 2006
